Calculate the Value of Kp for the Equation
H2g CO2g. If the Kc for the chemical equation below is 69 10-4 at a temperature of 250K then what is the Kp.

Solved Calculate The Value Of Kp For The Equation Given That Chegg Com
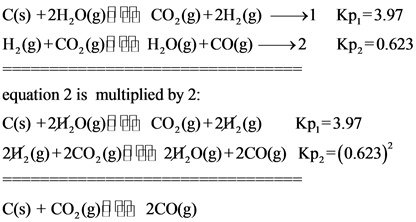
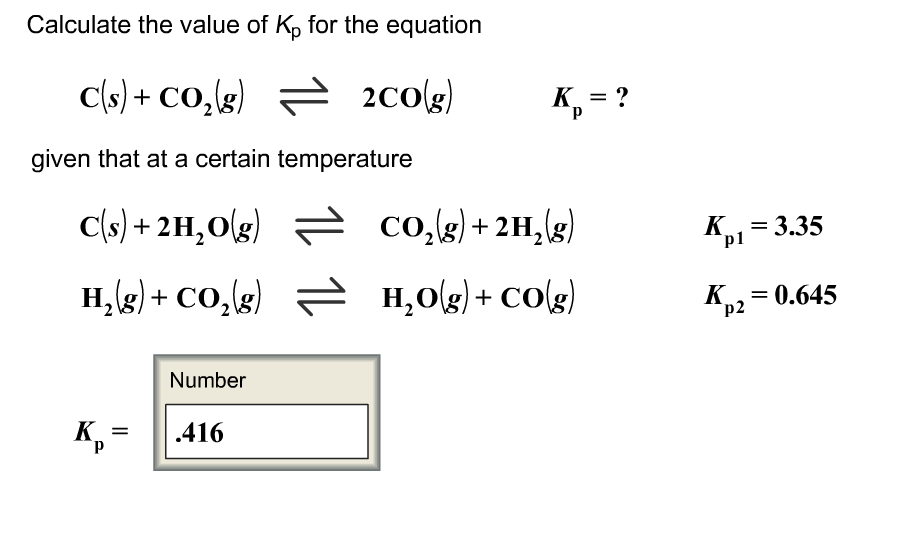
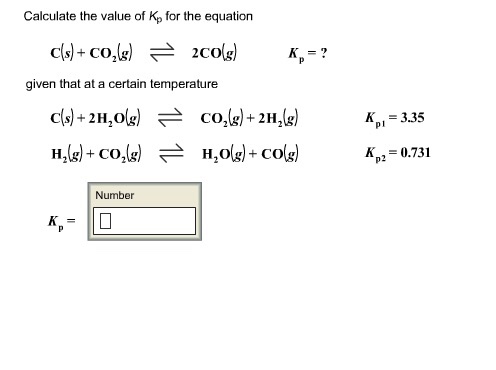
Calculate the value of Kp for the equation C sCO2 g2CO g𝐾pC sCO2 g2CO gKp.
. Use data sheet to find value for ideal gas constant R R 00821 Ideal Gas Constant Use balanced chemical equation to calculate Δn. Now by multiplying the coefficients of the reaction it also affects the value of Kp. The form of the temperature dependence can be taken from the definition of the Gibbs function.
Calculate the value of K for the equation Cs CO g 2COg Kp given that at a certain temperature Cs 2 H Og COg 2 H g Kp1 303 H g. Calculate the value of Kp for the equation. Because the value of Δ G r x m o is dependent on temperature the value of K p is as well.
Just like K c K p always has the same value provided you dont change the temperature irrespective of the amounts of A B C and D you started with. K P K c RT Δn. K p 227 10-2 00820574614 298-2 3796 10-5.
Choose units and enter the following. Kp Pproducts Preactants If we modify the coefficients by two Kp is altered. In general for any chemical reactions of gas molecules the relation between K p And K c is-.
Cs 2H2Og CO2g 2H2g Kp1 397. Finally calculate the value of K p for the equation. Calculate the value of Kp for the equation CsCO2g2COg Kp.
Given that at a certain temperature. Calculating an Equilibrium Constant Kp with Partial Pressures. The Conversion of Kc to Kp calculator uses the formula Kp Kc RTn-n0 to compute an equilibrium constant in terms of pressure by using an equilibrium constant in terms of molarity.
2H2g 2CO2g 2H2Og 2COg Kp 0601². Kc Equilibrium constant in terms of molarity molsL T Temperature n Number of moles of products in the gas phase. K c K p R T Δ n.
K p K c R T Δ n. Remember that Kp is a value that you can obtain by doing this. Given that at a certain temperature.
It is used to express the relationship between product pressures and reactant pressures. K p 696 x 10-500821333 2 0052. Calculate the value of Kp for the equation.
H 2 g CO 2 g H 2 Og COg K p2 0619. H 2 g CO 2 g H 2 O g CO g Kp 2 0623. K P K c RT Δn.
Kp 905 10-4. Estimate the value of K p for this system. Check to see that the given amounts are measured in appropriate pressure units since K p is to be.
Dn 2 moles of gaseous products - 0 moles of gaseous reactants 2. Given that at a certain temperature Cs2H2OgCO2g2H2g Kp1333 H2gCO2gH2OgCOg Kp2733 Please show detailed steps. Calculate the difference in the number of moles of gases Dn.
Kp Pprod² Preact². Calculate the value of K p for the equation. Substitute the values into the equation and solve for KP.
Cs 2H 2 Og CO 2 g 2H 2 g K p1 309. Δn 2 1 - 2 1. It is a unitless number although it relates the pressures.
Cs CO 2 g 2COg K p. K p pN 2pH 2 3pNH 3 2 α12α Pt 3α12α 3 12α12αPt 2 27α 4 Pt 2 12α 2 12α 2 27α 4 Pt 2 1 4α 2. Given that at a certain temperature C s2H2O gH2 gCO2 gCO2 g2H2 gH2O gCO g𝐾p1𝐾p23390625C s2H2O gCO2 g2H2 gKp1339H2 gCO2 gH2O gCO gKp20625 𝐾pKp.
K P 375 10 -6 00821 1069 1. Vapour Density and Number of Moles. 2 N 2 O 3g 2 N 2g 3 O 2g Answer.
Now you know the equilibrium constant for an example of the Haber process and how to calculate K p. Calculate the value of K p for the following reaction at 333 K. K p is the equilibrium constant calculated from the partial pressures of a reaction equation.
K p has exactly the same format as K c except that partial pressures are used instead of concentrations. Knowing Kp and the total pressure degree of dissociation of ammonia can be calculated. Write the equilibrium expression to find K p.
Kp 2x188x0311118 arpm 11000K 1882 x0311 i asecrpm The PI compensation can be represented as- s s K K K s K s K s K K V I i p i i p i p 1 2 s K2 t2s 1 i p K K t2 p i K K w2 Corner frequency 94 sec 117 11000 2 rad K K p ω i The frequency response for PI compensation is as follows. Substitute the values into the equation and calculate K p. C s CO 2 g 2 CO g Kp.
But if you dont feel like doing all that math on your own you can always put our K p calculator to good use. C s 2 H 2 O g CO 2 g 2 H 2 g Kp 1 397. K p pN 2pH 2 3 pNH 3 2.
It was found that the pressure of the nitrogen dioxide decreased by 0952 atm. Write the expression to convert K c to K p. From Vapour Density Measurements.
Given that at a certain temperature. Cs CO2g 2COg Kp. Cant get enough of chemistry.
BaOH 2 aq 2 HCl aq 2 H 2 O l BaCl 2 aq. If the Kp for the chemical equation below is 57 10-2 at a temperature of 273K then what is the Kc. The gases on the right-hand side of the chemical equation are at the top of the expression and those on the left at the.
Kp 86 10 3. At constant temperature and pressure.
Solved Calculate The Value Of Kp For The Equation Chegg Com

Calculate The Value Of Kp For The Equation C S Co2 G 2co G Kp Home Work Help Learn Cbse Forum
Solved Calculate The Value Of Kp For The Equation C S Chegg Com
No comments for "Calculate the Value of Kp for the Equation"
Post a Comment